Decision of the 21st Meeting of the Committee for
Drawing Up and Promoting the Action Program
The Government of Japan has voluntarily decided upon the "Measures related to Japanese Public Sector Procurement of Medical Technology Products and Service," as outlined herein.
MEASURES RELATED TO
JAPANESE PUBLIC SECTOR PROCUREMENT
OF MEDICAL TECHNOLOGY PRODUCTS AND SERVICES
I. GENERAL POLICIES
(1) domestic products, services and suppliers; and
(2) products, services and suppliers of any other foreign country.
(l) treat a locally-established supplier less favorably than another locally-established supplier on the basis of degree of foreign affiliation or ownership; or
(2) discriminate against a locally-established supplier on the basis that the products or services offered by that supplier for the particular procurement are foreign products or services.
III. POLICIES AND PROCEDURES APPLICABLE TO ALL PROCUREMENTS COVERED BY THE MEASURES
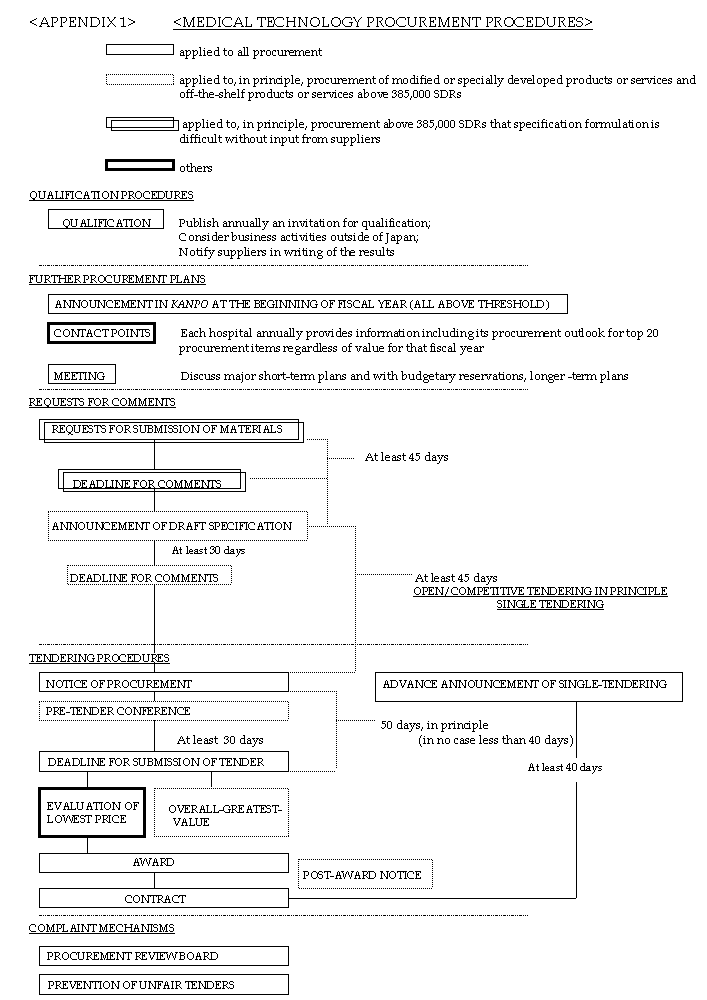
Entities will invite suppliers to submit comments on the following procurement plans by publishing in the Kanpo procurement information of medical technology products and services (the name and address of the entity, subject matter of the procurement such as its name and volume, planned date of the notice of procurement), covered by the Measures as early in the fiscal year as possible, and will make the information available for public perusal at a contact point in the entity, as provided for in Section VI(l). In the case that the notice of procurement or the Request for Comments set out in the sub-paragraph 5 below has been published, entities need not take the procedures to provide information set out in this paragraph.
2.1 Where an entity has a requirement for a medical technology product or service, it will undertake procurement planning and conduct market research, as necessary, in order to promote competition to the maximum extent possible, and to ensure that the entity meets its needs with the most appropriate medical technology product or service.
2.2 Information made available on a budget request to any suppliers will be
made available on a non-discriminatory basis. No entity may provide advance knowledge
to any supplier, if such knowledge would give that supplier an advantage over
other suppliers, about pre-tender information, where available, at any stage of
a procurement, from the formulation of a budget request and the beginning of specification
development through issuance of tender documentation to award of a contract. Entities
will accord equal access to all pre-tender information to all foreign and domestic
suppliers and provide them with equal opportunities to participate in
pre-tender activities. No entity may use information gathered during the pre-tender
phase to exclude any supplier.
2.3 Entities will ensure that all suppliers are given the same opportunities to participate in technical reference committees, advisory groups, study councils, and any such groups, if established, that discuss the technology, budget, specifications, functions or any other aspect of procurements of medical technology products and services.
2.4 Qualification of Suppliers
(1) Entities, in the process of qualifying suppliers in a tendering procedure, will not discriminate among foreign suppliers or between domestic and foreign suppliers.
(2) Entities will limit any conditions for participation to those that are essential to ensure the supplier's capacity to fulfill the contract in question.
(3) Entities will publish annually in the Kanpo an invitation to suppliers to qualify, which will set forth objective and specific qualification requirements for participation in tenders.
(4) In determining whether a supplier is qualified, entities will consider net worth and business activities outside of Japan.
(5) Entities will provide opportunities to suppliers to qualify at any time, including after a Notice of Procurement has been issued for a particular procurement. The qualification obtained will be effective until the next regular qualification. If qualified at a regular qualification, the qualification will be effective for at least two years.
(6) Entities will notify suppliers in writing of the results of the qualification. If the entity does not qualify a supplier, the entity will notify the supplier of the reasons for the disqualification and that it is entitled to request further explanation within seven days of receipt of the disqualification notice.
2.5 Entities will not award a contract for medical technology products or services to any supplier, or to its affiliates, if that supplier has provided research or design services for that procurement, and such involvement could result in an unfair competitive advantage, except to the extent such services are included in the contract for procurements requiring the Request for Comments procedures set out in the Measures.
2.6 Entities will treat follow-on contracts as separate procurements subject to the procedures set out in the Measures. Contracts awarded as the result of the exercise of options or renewal provisions in a contract awarded in accordance with the procedures set out in the Measures will not be considered "follow-on" contracts.
2.7 No entity may:
(1) prepare, design or otherwise structure any procurement with the intention of avoiding the application of the Measures or favoring any particular supplier; or
(2) divide a procurement with the intention of reducing the value of any resulting contracts below the threshold set out in Section I.
2.8 Entities will:
(l) determine the value of contracts consistent with the Code and the Measures, in determining whether a procurement is subject to the Measures.
(2) not select a valuation method for a proposed procurement with the intention of avoiding the application of the Measures.
3.1 Entities will use open tendering procedures, to the maximum extent possible, for the procurement of medical technology products and services.
3.2 The Government will ensure that the tendering procedures of its entities:
(1) are applied in a non-discriminatory manner;
(2) do not provide any supplier with information on a specific procurement in a manner that would have the effect of diminishing competition; and
(3) are consistent with the provisions of the Measures.
4.1 Entities will reduce their use of single tendering.
4.2 Because competitive procurements are the foundation of the Government's procurement policies and practices, single tendering will be used only in exceptional cases, justified in accordance with Code procedures, and will not be used to favor or exclude domestic or foreign suppliers of medical technology products or services, or to contravene any provision, intent or purpose of the Measures.
4.3 Except in the cases that no tenders are submitted in competitive tendering or no successful tenders are resubmitted in the second tendering, or in cases of extreme urgency, entities will publish an announcement of a single tender procurement covered by the Measures in the Kanpo at least 40 days before the contract is awarded. The notice will contain:
(l) a description of the procurement, including volume to be procured;
(2) the planned contract date;
(3) the Code justification for the single tender; and
(4) the name of an intended supplier, in the case that discussions on the contract have begun with that supplier.
5.1 Request for submission of materials
For the procurements in which entities face difficulties in developing appropriate specifications without requesting the submission of materials from suppliers, and the contract awards of which are expected to be greater than 800,000 SDRs, entities will take the following at the beginning of the fiscal year or as early as possible before the beginning of the fiscal year, except in the case of urgency or in the case of single tendering provided for in the Code:
(l) Entities will publish a notice in the Kanpo of their request for materials and other necessary information on basic needs of the planned procurement, and provide copies of the notice to suppliers upon request.
(2) The notice in the Kanpo includes the following:
(a) the name and address of the entity;
(b) subject matter of the procurement (its name and volume, basic needs of the planned procurement);
(c) deadline of the submission of the materials; and
(d) notice of a conference for the planned procurement, if such a conference is held.
(3) The deadline of (c) above will be, except in the case of urgency, at least 45 days after publication of the Request for submission of materials.
(4) Where an entity amends or has additional information concerning an announced procurement set out in (2) above, it will simultaneously provide the amendment or additional information to all suppliers that have responded to the Request for submission of materials. If the amendment or additional information are concerning the subject matter of the procurement set out in (b)above, entities will allow at least 30 days for suppliers to consider and respond to the amendment or information.
5.2 Request for Comments on Draft Specifications
For (i) procurements of modified products or services or specially developed products or services, (ii) procurements of all off-the-shelf products or services with a value greater than 800,000 SDRs, or (iii) other procurements for which entities acknowledge the need to use the Request for Comments, entities will take the following measures in order to ensure that interested suppliers submit their comments on draft specifications prepared by the entities. In the case of urgency, however, the entities may shorten the period to the extent that suppliers will be able to respond, by announcing specific reasons for the period reduction in the notice of the Request for Comments in the Kanpo. In the case of extreme urgency with which the entities will not be able to cope by the above period reduction, the entities may omit part or whole of the procedures set out below, provided that the entities announce specific reasons for the omission in the Notice of Procurement.
(l) Entities will publish the notice of the completion of developing draft specifications in the Kanpo at least 45 days before the intended date of the Notice of Procurement, and will promptly send a copy of the Request for Comments to suppliers upon request.
(2) Entities will announce the following in the notice of the completion of developing draft specifications:
(a) subject matter of the procurement (its name and volume);
(b) the addresses from which the draft specifications may be obtained;
(c) the deadline for the submission of comments;
(d) the name and address of the entity; and
(e) the date and location of the conference for draft specifications, if such conference is to be held.
(3) The deadline for the submission of comments set out in (c) above will be at least 30 days after the day following the publications of the Request for Comments for draft specifications.
(4) When entities recognize the need to improve their draft specifications announced in the notice of the Request and amend them as a result of the comments submitted from interested suppliers, the entities will notify all the domestic and foreign suppliers that have expressed interest in the procurement. In such a case, the entities will allow sufficient time for the deadline for the submission of comments in order for suppliers to consider and respond to the amendment or information prior to publication of the Notice of Procurement.
6.1 Any technical specification prescribed by an entity will be, where appropriate:
(l) specified in terms of performance rather than design or descriptive characteristics; and
(2) based on international standards, where such exist, and otherwise based on national technical regulations or recognized national standards.
6.2 Entities will prepare technical specifications with the minimum detail needed to define the performance criteria. Entities will not require features not essential to the performance criteria.
6.3 Entities will formulate specifications in an impartial manner. Entities will not prepare, adopt or apply any technical specification with the intent of creating obstacles to any supplier or class of suppliers, including foreign suppliers. If the procurement will replace or interconnect with an existing system, the specifications will not be designed to preclude competition.
6.4 Entities will not allow any supplier directly involved in the development of specifications in a procurement to participate in the tendering process, except where:
(l) the supplier has provided comments in response to a Request for Comments, as provided for in Section III(5) and such participation would not result in an unfair competitive advantage for any supplier;
(2) the supplier has provided information or assistance to an entity in preparing or refining specifications and the entity has controlled the process and conducted it in a fair and impartial manner and has provided the same opportunities to all suppliers to provide information and assistance; or
(3) the supplier has provided, at the request of an entity, product specifications or data about a product it supplies and all suppliers are provided an equal and impartial opportunity to participate or provide product specifications or data.
6.5 Entities will not prescribe a technical specification that requires or refers to a particular trademark or brand name, patent, design or type, specific origin or producer or supplier unless there is no other sufficiently precise or intelligible way of describing the procurement requirements and provided that, in such cases, words such as "or equivalent" are included in the tender documentation.
7.1 Entities will invite all suppliers to participate in the procurement by publishing in the Kanpo a Notice of Procurement at least 50 days, in principle, but in no case less than 40 days, prior to the deadline for the submission of tenders, unless justified by the Code.
7.2 Each entity will, after publishing the Notice of Procurement in the Kanpo, promptly make such Notice available for public perusal at a contact point in the entity, as provided for in Section VI(l).
7.3 The Notice of Procurement will include sufficient information for a supplier to make an informed decision as to whether to participate in the procurement, and will contain the following information:
(1) subject matter of the procurement;
(2) method of evaluation of tenders;
(3) the addresses from which the tender documentation may be obtained;
(4) if a pre-tender conference is held, its date and location; and
(5) the deadline and address for the submission of tenders:
7.4 If after publication of the Notice of Procurement, but before the deadline for submission of tenders, the entity amends the Notice, it will publish the amendment in the Kanpo and make the information available for public perusal at a contact point in the entity, as provided for in Section VI(1).
8.1 Entities will use tender documentation to communicate their needs to suppliers and to solicit tenders from them.
8.2 Entities will prepare the tender documentation, including evaluation criteria when the overall greatest value methodology is used, in an impartial manner so as to ensure that equal opportunities are provided to all suppliers on a non-discriminatory basis.
8.3 In preparing tender documentation, no entity may accept the provision of any assistance from any supplier, which could give that supplier any advantage over other suppliers, other than in accordance with the procedures set out in the Measures.
8.4 Tender documentation provided to suppliers will contain all information necessary to permit them to submit responsive tenders, including information required to be published in the Notice of Procurement, except for the amount and terms of payment of any sum payable for the tender documentation, and the following:
(1) the address of the entity to which tenders should be sent and the names of officers responsible for the procurement;
(2) the address to which requests for supplementary information should be sent;
(3) the language or languages in which tenders and other tendering documents must be submitted;
(4) the closing date and time for receipt of tenders, and the length of time during which any tender should be open for acceptance;
(5) the persons authorized to be present at the opening of tenders and the date, time and place of the opening;
(6) any economic and technical requirements, financial guarantees and other information required from suppliers;
(7) a complete description of the products or services to be procured and requirements, including technical specifications, conformity certification and necessary plans, drawings and instructional materials;
(8) all criteria that will be applied to determine the successful supplier that will be awarded the contract, including all evaluation factors and sub-factors, weighted in terms of importance to the evaluation and including any factors that are to be considered and the cost elements to be included in evaluating prices, such as transportation, insurance and inspection costs;
(9) the terms of payment;
(10) if a pre-tender conference is held, its date, time and location; and
(11) any other terms or conditions.
8.5 Entities will:
(1) make the tender documentation available at the time of publication of the Notice of Procurement;
(2) send the tender documentation promptly to a supplier upon its request;
(3) reply promptly to any reasonable request for information relevant to the tender documentation made by a supplier participating in the tendering procedures, on the condition that such information does not give that supplier an advantage over its competitors in the award of the contract;
(4) promptly put in writing communications with suppliers, except when it imposes an unnecessary burden on the entity, concerning the preparation of tender documentation, including specifications, standards and other tendering terms.
9.1 At least 30 days prior to the deadline set out in the Notice of Procurement for the submission of tenders, entities will hold a pre-tender conference with regard to any procurements for which Request for Comments procedures are to be taken as set out in Section III(5) and for any other procurement, as necessary. Such conferences will include the opportunity for direct discussions between suppliers and that entity on technical, administrative and any other aspect of the procurement, and for all suppliers to obtain information on tendering on an equal basis.
9.2 Entities will not make attendance at a pre-tender conference a pre-requisite for tender submission or consider attendance in the evaluation of tenders.
10.1 In evaluating tenders and selecting the successful supplier, entities will use a selection procedure designed to:
(1) maximize competition;
(2) minimize the complexity of the tender documentation, the evaluation and the selection decision; and
(3) ensure impartial and comprehensive evaluation of tenders submitted by suppliers.
10.2 Entities will evaluate tenders in a transparent manner that ensures equal treatment of all suppliers submitting tenders. Where an entity conducts technical evaluations, it will conduct them under the same conditions for all suppliers in the tendering process and will apply the same testing criteria, which will be made immediately available to suppliers on request.
10.3 No entity will refuse to consider as a responsive tender any offer by a supplier to supply a product that is used, or accepted for use, in any Special Function hospital or other hospital or laboratory as part of the Highly-Advanced Medical Technology program, if the product meets the specifications set out in the tender documentation.
10.4 Entities will evaluate tenders as follows:
(1) After a one-year preparation period following introduction of the Measures, entities will evaluate tenders and award contracts based on the overall greatest value for the entities, for (i) procurements of modified products or services or specially developed products or services; or (ii) procurements of off-the-shelf products or services with a value greater than 800,000 SDRs. For other procurements, entities may use the overall greatest value methodology based on their own decision.
(2) Tenders will be evaluated on a pass/fail basis based upon the specific technical and other evaluation criteria stated in the specifications, and contracts will be awarded to the lowest-priced tender among those tenders which have met the evaluation criteria, unless the entity chooses the overall greatest value methodology as set out in subparagraph (l) above.
10.5 Where evaluation of tenders is conducted based on the overall greatest value methodology, the following procedures will apply:
(l) Entities will evaluate tenders based on overall greatest value to the entity, which is determined by considering functional and performance factors, price and other factors specified in the tender documentation. Entities will apply the relative weights set out in the tender documentation to the evaluation criteria. The price/cost evaluation may be based on the total life cycle cost of procurement.
(2) Entities may require clinical trials of prototypes as part of the evaluation process leading to the award of the contract, provided that this requirement is set out in the tender documentation and the clinical trials are conducted in a fair and impartial manner.
(3) When entities use the overall greatest value methodology, they may not change the evaluation factors and their relative importance for a specific procurement without formally amending the tender documentation and providing the amended tender documentation in the same manner to the same suppliers as the original tender documentation.
(4) Entities will make the award as soon as practicable after completion of the evaluation process.
(5) Entities will put in writing promptly evaluation of tenders and the resulting selection decision, including the scoring of all factors and name of persons responsible for selection decisions.
10.6 No supplier will be allowed to modify the contents of a tender once submitted.
11.1 The entity will make the award as soon as practicable after completion of the evaluation process and will publish information on the contract award in the Kanpo and promptly notify all suppliers that submitted tenders of its selection and the award price and will also make the information available for public perusal at a contact point in the entity, as provided for in Section VI(l).
11.2 Upon request from an unsuccessful supplier, an entity will promptly provide such supplier with an explanation of the reasons for not being selected, the name of the selected supplier and the relative advantages of that supplier's tender where the overall greatest value methodology is used.
11.3 Including those cases in which entities provide information in accordance with paragraph 11.2, entities will not:
(l) disclose to any third party a supplier's trade secrets, manufacturing process, intellectual property or other confidential business information provided by a supplier; or
(2) supply to any third party information that would prejudice the legitimate commercial interest of a supplier or fair competition among suppliers.
Any modification of the scope of a contract that would increase the value of the contract by more than 10 percent of its value will be subject to the provisions of the Measures as if it were a new procurement.
IV. REGULATORY REQUIREMENTS
V. SUPPORTING MEASURES
Entities will make the maximum possible use of procedures described in 6. of the Procedures for Government Procurement on Products (Operational Guidelines) to contribute to convenience for domestic and foreign suppliers that have expressed an interest in government procurement of medical technology products and services.
To ensure effective implementation of the Measures, the Government will set up a forum for follow-up to examine concrete steps including the following.
2.1 The Government will establish a committee to study standardized manual to develop non-discriminatory and simplified specifications for procurements of medical technology products and services procured by two or more entities.
2.2 The Government will establish a committee to develop a standardized format, consistent with the Measures, to be used by all entities, to the extent practicable, for tender documentation of medical technology products and services.
2.3 Training
The Government will establish a program to provide training for entity procurement officials regarding the implementation of the Measures.
Where the Government establishes any committee or similar groups, whether formal or informal, which includes only private sector or both public and private sector participation, primarily related to the public sector procurement of medical technology products or services, the Government will publish notice in the Kanpo of information related to the group's establishment.
VI. PROVISION OF INFORMATION FOR ALL PROCUREMENTS REGARDLESS OF VALUE
For all procurements of medical technology products and services by the entities, without regard to the value of the procurement, the Government will take the following actions:
4.1 Each entity will conduct an annual conference for entity procurement officials and domestic and foreign suppliers to discuss information about the entities' major short-term procurement plans and, with budgetary reservations, their longer-term procurement outlook. This may be replaced by the entity's participation in a similar conference established by the Government or their entities.
4.2 Each entity conducting a procurement conference will publish notice of the conference in the Kanpo at least 30 days prior to the conference.
(1) advise officials involved in the implementation of procurement to meet with domestic and foreign suppliers, on request and to be responsive to their questions and concerns; and
(2) ensure that such officials do not provide preferential access to domestic or foreign suppliers.
VII. UNFAIR TENDERS
VIII. COMPLAINT MECHANISMS (Integrated See I-4)
Procedures of fair complaint mechanisms, described in Annex 4 of the Action Plan on Reform of the Bidding and Contracting Procedures for Public Works (hereinafter referred to as the "Action Plan"), will be applied to provide equitable, timely, transparent and effective complaint procedures for suppliers of medical technology products and services covered by the Measures, until the new Agreement on Government Procurement enters into force and Japan becomes a party to it. The title "construction procurement review board" will be replaced by "procurement review board." In light with the nature of medical technology products and services, the following modifications will be made. (For ease of reference, Annex 4 of the Action Plan, as amended by the following, is attached as Annex 3 (Omitted) of the Measures.)
IX. ENCOURAGEMENT TO PREFECTURAL GOVERNMENT AND DESIGNATED CITIES
The Government will encourage prefectural governments and Designated Cities to take, in principle, necessary measures similar to the Measures, for their procurement of not less than 200,000 SDRs, taking into account local circumstances and the provisions of relevant laws and regulations.
The Government will encourage prefectural governments and Designated Cities to consider establishing a review mechanism with respect to their procurement of not less than 200,000 SDRs.
X. TIME TABLE FOR IMPLEMENTATION
The Measures will be basically introduced to the maximum extent for procurement under the initial budget of FYl994, and a system for procurement in accordance with the Measures will be established by the end of FYl994.
XI. REVIEW OF THE IMPLEMENTATION OF THE MEASURES
The Government will hold a review to assess the extent that the Measures contribute to improvement of non-discriminatory nature, transparency, openness, competitiveness and fairness of procurement of medical technology products and services covered by the Measures and, in addition, to address specific issues in implementing the Measures. The review will be held annually, and as necessary, under the Committee for Drawing Up and Promoting the Action Program. Administration relating to the review will be governed by the Cabinet Councillors' Office on External Affairs. At the review, implementation and utilization by suppliers of the Measures will be examined by using statistics and other relevant information. This will include the opportunity of listening to opinions of domestic and foreign companies and business associations.
XII. DEFINITIONS
For purposes of the Measures:
"Days" mean calendar days, except in the case of Annex 3, where references to days means working days;
"Locally-established supplier" means a supplier that is established in Japan, without regard to the source of its capital;
"Medical technology products" means medical instruments and apparatus, medical supplies and dental materials, excluding these for animal use, listed in Annex l of the Enforcement Ordinance of the Pharmaceutical Affairs Law;
"Medical technology services" means design services of medical technology products, and design services of software which is solely used in medical technology products;
"Supplier" means a person that has provided or could provide products or services in response to a Notice of Procurement;
"Affiliates" mean (a) companies which a supplier who has provided research or design services controls or are controlled by, or (b) other companies which are controlled by a company controlling a supplier who has provided research or design services, where control means ownership in excess of 50% of the issued stock if the affiliate is a stock corporation, and ownership in excess of 50% of the capital if the affiliate is a limited company;
"Modified products or services" means medical technology products or services that exist in the international marketplace at the time the Request for Comments is published in the Kanpo but require modification to meet the legitimate requirements of the entity for the procurement that substantially transform their function or essential physical characteristic;
"Off-the-shelf-products or services "means medical technology products or services that exist in the international marketplace at the time the Request for Comments or the Notice of Procurement is published in the Kanpo; and
"Specially developed products or services" means medical technology products or services that do not exist in a form that meets the performance requirements in the international marketplace and must be developed especially to meet the legitimate requirements of the entity for the procurement.
ANNEX l
CENTRAL GOVERNMENT ENTITIES
ANNEX 2
OTHER ENTITIES COVERED BY THE MEASURES
iNote: The entities above are listed only under the names they held as of February 1, 2013, and the names of special corporations before their merger or abolition are not given.j